INTACT
RetINal Toxicity And HydroxyChloroquine Therapy StudyThe INTACT study is a 5-year longitudinal study that is following Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE) patients in BC who are currently taking and have been taking Hydroxychloroquine (HCQ) for ≥5 years, with the goal of finding the risk of retinal toxicity as a result of long term HCQ therapy.
Study Start Date: 2021 Study End Date: 2027
Principal Investigator:
J. Antonio Aviña-Zubieta, MD, MSc, PhD, FRCPC
Senior Scientist, Arthritis Research Canada
Division Head & Associate Professor, Division of Rheumatology, Department of Medicine, University of British Columbia
For a full list of team members, see below.
Why do this research?
Systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) are diseases of the immune system that affect many parts of the body (e.g., joints, heart, eyes, skin), and can result in other medical complications and death. A drug called hydroxychloroquine (HCQ) is commonly used to treat SLE and RA. Existing studies have highlighted benefits of HCQ, including less severe disease, fewer complications (e.g., blood clots, problems with pregnancy or kidneys), and longer life. Although HCQ is typically safe, inexpensive and easy to take, a serious long-term side-effect that may occur after years of taking HCQ is eye damage that could cause vision loss. Given the benefits and common use of HCQ, it is critical that the real risk of eye damage be determined and findings shared with doctors and patients so that patients receive the best and safest care.
What will be done?
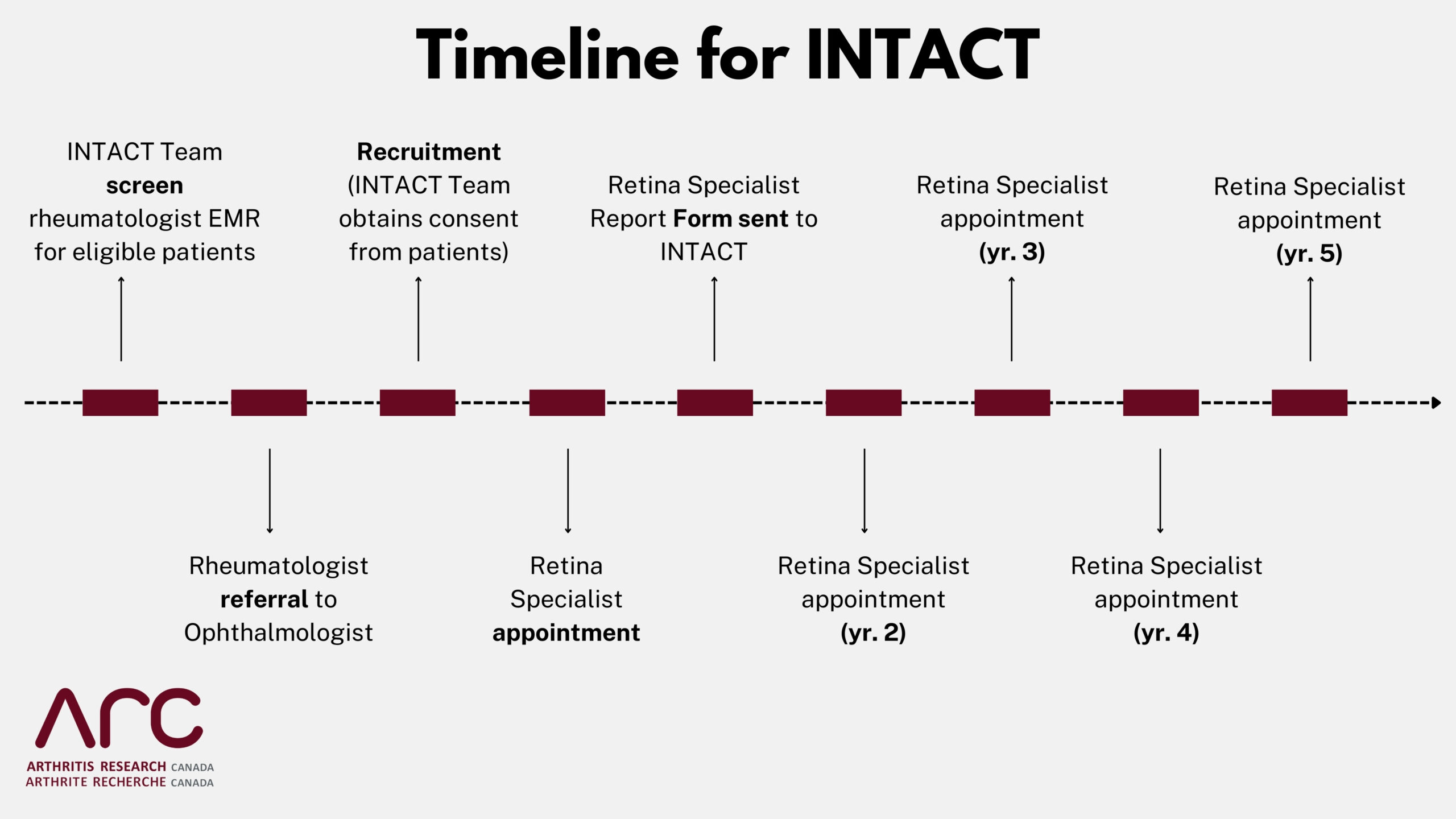
Using administrative health data, we will identify all adults in BC with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) who have been taking hydroxychloroquine (HCQ) for at least 5 years. People who are interested in participating will have state-of-the-art eye testing done at a retinal specialist’s office, which is already the standard of care for people taking HCQ. This eye testing will take place once per year for 5 years. The goal of this study is to determine the risk of eye damage in people with SLE and RA, who are taking HCQ.
Who is involved?
The research team consist of researchers, rheumatologists, retinal specialists (a type of ophthalmologist) and family physicians who are committed to delivering safe and appropriate care to the population.
Co-Principal Investigator
John Esdaile, MD, MPH, FRCPC Scientific Director Emeritus, Arthritis Research Canada; Professor, Division of Rheumatology, Department of Medicine, University of British Columbia
Co-Investigators
Hyon Choi, MD, DrPH, FRCPC Research Scientist, Arthritis Research Canada; Professor, Harvard Medical School
Linda Li, BSc(PT), MSc, PhD Senior Scientist, Arthritis Research Canada; Professor, Department of Physical Therapy, University of British Columbia
Hui Xie, PhD Research Scientist, Arthritis Research Scientist; Professor, Faculty of Health Sciences, Simon Fraser University
Diane Lacaille, MD, MHSc, FRCPC Scientific Director, Arthritis Research Canada; Professor, Division of Rheumatology, Department of Medicine, University of British Columbia
Kamran Shojania, MD, FRCPC Clinical Trialist, Arthritis Research Canada; Clinical Professor and Head, Division of Rheumatology, Department of Medicine, University of British Columbia
Mahyar Etminan, PharmD, MSc Associate Professor, Department of Ophthalmology and Visual Sciences, Faculty of Medicine, University of British Columbia
David Maberley, MD Chairman, Department of Ophthalmology, University of Ottawa; Head, Department of Ophthalmology, The Ottawa Hospital
Martin Dawes, MBBS, MD, FRCGP Professor Emeritus, Department of Family Practice, Faculty of Medicine, University of British Columbia
Collaborators
Alison Hoens, PT Knowledge Broker, Arthritis Research Canada; Clinical Professor and Knowledge Broker, Department of Physical Therapy, University of British Columbia
Cheryl Koehn, President, Arthritis Consumer Experts
Research Staff
Narsis Daftarian, MD Research Assistant, Arthritis Research Canada; PhD Candidate, Department of Experimental Medicine, University of British Columbia
Jeremiah Tan, BSc; Research Assistant, Arthritis Research Canada
Dora Chan, BSc Candidate; Research Assistant, Arthritis Research Canada
Ayesha Kirmani, MD; Research Assistant, Arthritis Research Canada
Who is funding this research?
Canadian Institutes of Health Research
How do people get involved?
Your participation in this study is voluntary and there are no foreseeable risks that are associated with this study. You will be asked to fill out a consent form before participating.
Your participation would involve signing a consent form and seeing a retinal specialist once per year for five years for eye exams. These exams are already the standard of care for people with systemic lupus erythematosus or rheumatoid arthritis who are taking hydroxychloroquine.
If you receive a letter of invitation in the mail, please consider participating in our study!
You may be eligible if you:
• Are an adult (18 years and older)
• Live with systemic lupus erythematosus or rheumatoid arthritis
• Have been taking the medication hydroxychloroquine for 5 or more years
• Live in British Columbia