Canadian Research Group of Rheumatology in Immuno-Oncology (CAN-RiO)
Why do this research?
Immunotherapy medications are revolutionizing cancer treatment. They work by ‘repurposing’ the immune system to fight cancer. Unfortunately, when the immune system is turned on to fight cancer, it can also lead to autoimmune diseases such as arthritis, skin rashes, endocrine disorders, and bowel inflammation. Because these autoimmune diseases are caused by the immune activation as a result of medication they are called, immune-related adverse events (irAE). Those specific to rheumatic disease are often termed rheumatic-irAE or Rh-irAE.
Since immunotherapy medications have been introduced only recently, there are no standardized guidelines on how to best treat these autoimmune side effects. Patients are highly motivated to continue their immunotherapy, as it works well for treating cancer, yet we don’t know if these autoimmune side effects are transient or if they will become chronic in nature. Furthermore, if we treat their autoimmune side effects with medications which suppress the immune system, it is possible that this will negate the beneficial anti-cancer immunotherapy effects and make the cancer worse?
Finally, patients with pre-existing autoimmune conditions have been generally excluded from the cancer studies evaluating these new therapies. As such, we don’t have information on how to manage their underlying disease, and whether immunotherapy is safe in these patients.
Understanding the mechanisms behind these immune side effects may help us identify strategies to reduce them and allow patients to continue on these life-saving drugs. It also may help us better understand traditional auto-immune diseases not caused by the immunotherapy.
Who is involved?
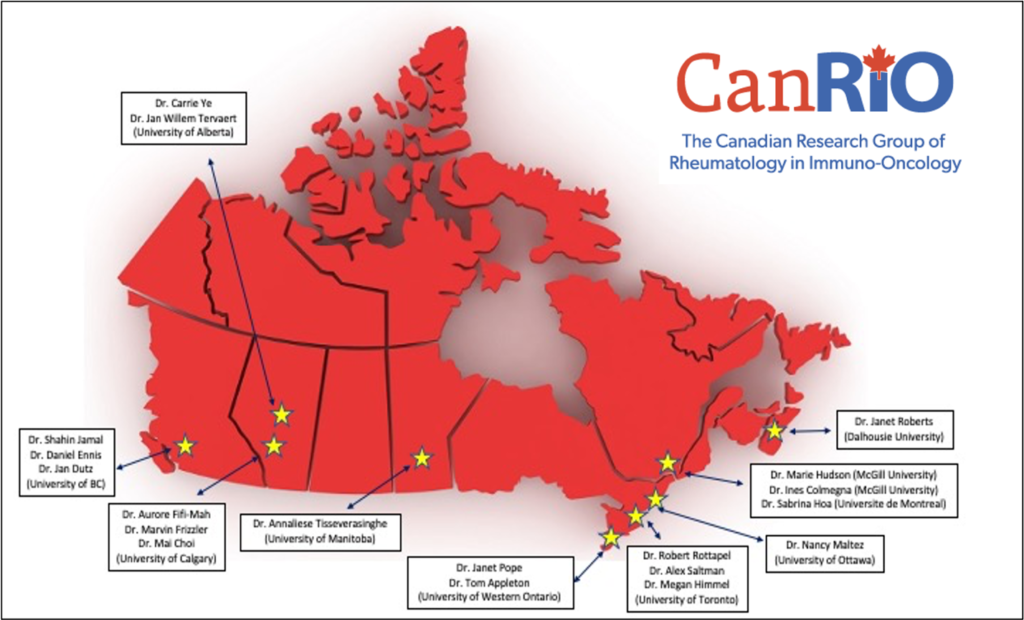
The Canadian Research Group of Rheumatology in Immuno-Oncology (CAN-RiO) is a network of 19 rheumatologists across Canada (see map) with expertise in the assessment and management of rheumatic complications of cancer immunotherapy. It includes clinicians, basic scientists, immunologists and epidemiologists from most of the major academic centers in Canada including University of BC, University of Calgary, University of Alberta, University of Manitoba, University of Toronto, University of Western Ontario, University of Ottawa, McGill University, and Dalhousie University.
All CanRiO sites are academic referral centers where investigators are local champions for the assessment and management of patients that (1) have developed de novo autoimmune disease after exposure to immunotherapy or (2) have pre-existing autoimmune disease and are being considered to receive immunotherapy for cancer.
What will be done?
CanRiO’s mission is to improve the care of cancer patients across Canada receiving immunotherapy, who develop rheumatic complications or who have pre-existing autoimmune conditions, through collaboration, education and research.
This will be done through multiple projects including:
- The development of a prospective, longitudinal cohort of patients who develop toxicities that are followed over time. We will be collecting the clinical data on patients to see how they respond to different therapies. We will also be collecting blood samples so that we can evaluate if there are any proteins or other markers to predict how people will respond clinically.
- The maintenance of a website (https://canrio.ca/) for education and sharing of scientific information. Through the website, we have developed teaching modules, difficult case rounds and up-to-date information on advances in immunotherapy toxicities and their management.
- The development of clinical consensus statement based on the latest evidence to provide Canadian perspective on the best practices for assessment and management of patients who develop Rh-irAE as well as those with pre-existing autoimmune disease for whom immunotherapy is indicated.