How Safe and Immunogenic is the Moderna Vaccine in People with Rheumatic Diseases?
Scientific Study Title:
COVID-19 Vaccine in Immunosuppressed Adults with Autoimmune Diseases
Study Start Date:
2021
End Date:
2022
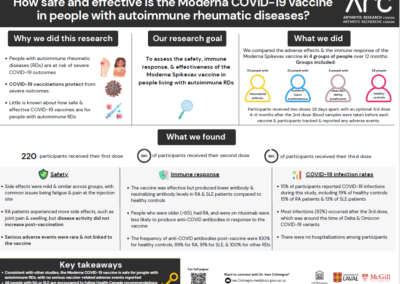
Why Did We Do This Research?
People with autoimmune rheumatic diseases, such as rheumatoid arthritis and systemic lupus erythematosus are at higher risk for severe infections. The immune systems of these patients are often suppressed due to their disease or its treatments, which could also impact how well vaccines work. Since the initial vaccine trials had excluded immunosuppressed patients, we evaluated the safety and immunogenicity (ability to generate anti-Covid antibodies) of the Moderna Spikevax vaccine in these patients. These were key questions for which there were no data at the time the study was designed.
What Did We Do?
We conducted a year-long study involving 220 participants, including adults with:
- Rheumatoid arthritis (131 patients),
- Systemic lupus erythematosus (23 patients),
- other rheumatic diseases using prednisone (8 patients), and
- healthy controls (58 people)
Participants received three doses of the Moderna Spikevax COVID-19 vaccine. We conducted evaluations on safety, generation of antibodies in response to vaccines, and COVID-19 infection rates. We compared these results between the rheumatic disease groups and the healthy controls, looking at:
- self-reported adverse events,
- levels of anti-Spike antibodies,
- cellular immune responses to Spikevax, and
- reported COVID-19 infections.
What Did We Find?
- Safety: The vaccine was safe across all groups. Adverse events were similar among participants with rheumatoid arthritis, lupus, and healthy controls. The rheumatoid arthritis patients reported more musculoskeletal side effects, such as joint pain and muscle pain. No serious vaccine-related adverse events were noted, and no increase in rheumatic disease activity was observed.
- Degree of vaccine response (Immunogenicity): The vaccine was less effective in generating anti-Spike antibodies in patients with rheumatoid arthritis and lupus compared to healthy controls. Specifically, patients with rheumatoid arthritis and lupus had lower levels of antibodies and neutralizing antibodies. Factors such as being over 65 years of age, and treatment with rituximab, were associated with reduced vaccine responses in rheumatoid arthritis patients.
- COVID-19 Infection Rates: The number of self-reported cases of COVID-19 among rheumatoid arthritis patients was the same to that in healthy controls.
The Moderna Spikevax vaccine is safe in people living with autoimmune rheumatic diseases. The response to the vaccine is very good in most patients but reduced in those over age 65 and in patients on specific treatments (i.e. rituximab).
What Are the Next Steps?
Following this study, we conducted a trial to evaluate strategies to enhance responses to COVID vaccines in patients on rituximab.
Infographic
Click to enlarge image
Research Team
Principal Investigators:
Ines Colmegna, MD, Research Scientist, Arthritis Research Canada (McGill University)
Paul R. Fortin, MD, MPH, FRCPC, Senior Scientist, Arthritis Research Canada, (Université Laval)
Co Investigators:
Sasha Bernatsky, MD, MSc, PhD (McGill University)
Brian Ward, MD, DTM&H, MDCM, (McGill University)
Louis Flamand, PhD, MBA (Université Laval)
Marc Dionne, MD, MPH, FRCPC (Université Laval)
Funding Agency:
Province of Quebec, Ministry of Health